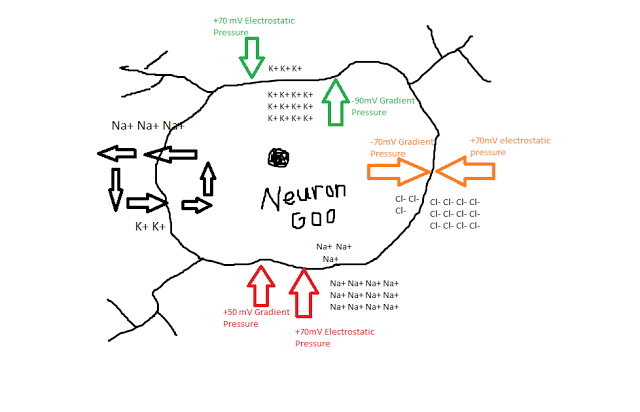
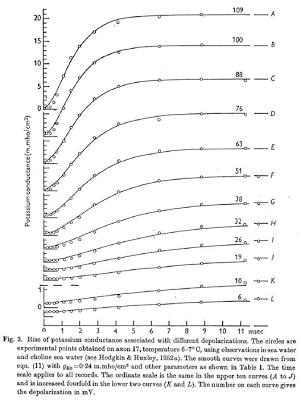
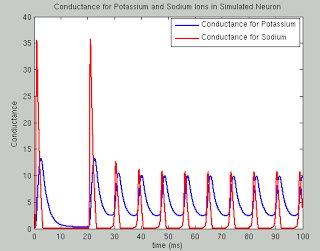
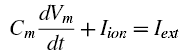Scientists, and neuroscientists in particular, are an odd bunch, with complex, multifaceted personalities. on the one hand they can be reclusive, socially awkward, and pretentious; but, in their defense, they make an honest effort to try and make up for these defects by being completely nuts.
For example, although most neuroscientists won't admit it, deep down, in their heart of hearts, somewhere in the left atrium, each and every one of feels a little twinge of excitement at the prospect of sticking electrodes somewhere in a person's brain and delivering electrical shocks. Seriously. Just ask any self-described neuroscience researcher what he would love to do most, and nine times out of ten he will say "Stick things inside someone's brain and inject enough electricity into it to light up a small amusement park." Only rarely will he give a more reasoned, more mature answer, such as "Purchase a motorcycle," or "Become an adult film star."
In any case, neuroscientists are usually prevented from acting out their sick fantasies by institutional review boards, or IRBs, which, from a neuroscientist's point of view, exist solely to be squeamish buzzkills and to put your experiments under review for a length of time equivalent to the gestation period of a yak. However, every once in a while there will be a case where an epileptic patient is undergoing a craniotomy, for example, or where a fellow neuroscientist is receiving extensive brain surgery after his latest motorcycle accident; and these cases, in addition to being like the Irish Sweepstakes for neuroscientists, can also yield valuable insights about how direct stimulation of cortical and subcortical areas can induce different physiological and cognitive states.
A recent example of this type of research appeared in a paper in the journal Neuron a couple of months ago, by Parvizi et al. Two epilepsy patients had deep-brain electrodes implanted in their brain, and the researchers were particularly interested in those electrodes located within the midcingulate region of the anterior cingulate cortex (ACC). After delivering small bursts of electricity to these electrodes, the patients reported higher levels of autonomic system activity, including increased heart rate and alertness, along with a feeling of foreboding but a concomitant feeling of resolve to overcome the intangible "challenge" that they felt. A follow-up resting-state analysis showed that both of these seed stimulation regions were hubs of a widespread cingulo-opercular network, similar to the typical coactivation of cingulate and insula responses observed in most studies examining the medial prefrontal cortex, and also involved in detecting emotional salience and sustaining goal-directed activity.
The authors labeled these feelings of wanting to overcome a formidable challenge as the "will to persevere," a phrase I think will be variously interpreted, but which seems apt enough for the current paper. However, one concern that popped into my head while reading through the article (dons reviewer glasses, purses lips disapprovingly) was: Is it really a will to persevere, or just a general increase in autonomic nervous system arousal (i.e., the sympathetic branch)? The "will to persevere" reported here may be the patient's interpretation of his increased heart rate, which, given the circumstances of the experiment and the patient's undergoing surgery to treat his epilepsy, may reflect his desire for a successful outcome of the surgery. Placing the patient in a different environment or with different circumstances - say, locking him in a room with one of the facehuggers from the movie Alien - may lead to a reinterpretation of the same increased arousal as fear, instead of a general willingness to overcome the challenge that lays in front of him.
In any case, these results, coupled with the lack of emotional response to electrical charges delivered to control stimulation sites and sham stimulations, lends support to the theory that the midcingulate region plays some kind of role in motivation, and that stimluation to this region may have practical applications for disorders involving pathologically low amounts of motivation, such as major depression and senioritis; disorders which, I might add, I am fully qualified to treat with open-brain surgery and a homemade electrical stimulation kit consisting of copper wire and a couple of lemons. Just give me a call.
Link to paper (including video of interview with subject 1; scroll to bottom of page): http://www.sciencedirect.com/science/article/pii/S0896627313010301
For example, although most neuroscientists won't admit it, deep down, in their heart of hearts, somewhere in the left atrium, each and every one of feels a little twinge of excitement at the prospect of sticking electrodes somewhere in a person's brain and delivering electrical shocks. Seriously. Just ask any self-described neuroscience researcher what he would love to do most, and nine times out of ten he will say "Stick things inside someone's brain and inject enough electricity into it to light up a small amusement park." Only rarely will he give a more reasoned, more mature answer, such as "Purchase a motorcycle," or "Become an adult film star."
In any case, neuroscientists are usually prevented from acting out their sick fantasies by institutional review boards, or IRBs, which, from a neuroscientist's point of view, exist solely to be squeamish buzzkills and to put your experiments under review for a length of time equivalent to the gestation period of a yak. However, every once in a while there will be a case where an epileptic patient is undergoing a craniotomy, for example, or where a fellow neuroscientist is receiving extensive brain surgery after his latest motorcycle accident; and these cases, in addition to being like the Irish Sweepstakes for neuroscientists, can also yield valuable insights about how direct stimulation of cortical and subcortical areas can induce different physiological and cognitive states.
A recent example of this type of research appeared in a paper in the journal Neuron a couple of months ago, by Parvizi et al. Two epilepsy patients had deep-brain electrodes implanted in their brain, and the researchers were particularly interested in those electrodes located within the midcingulate region of the anterior cingulate cortex (ACC). After delivering small bursts of electricity to these electrodes, the patients reported higher levels of autonomic system activity, including increased heart rate and alertness, along with a feeling of foreboding but a concomitant feeling of resolve to overcome the intangible "challenge" that they felt. A follow-up resting-state analysis showed that both of these seed stimulation regions were hubs of a widespread cingulo-opercular network, similar to the typical coactivation of cingulate and insula responses observed in most studies examining the medial prefrontal cortex, and also involved in detecting emotional salience and sustaining goal-directed activity.
 |
| Figure 1 from Parvizi et al, showing the stimulation site in the midcingulate region for both patients, as well as more remote stimulation sites for comparison. |
 |
| Figure 2 from Parvizi et al depicting a resting-state functional connectivity analysis using the midcingulate as a seed region. |
The authors labeled these feelings of wanting to overcome a formidable challenge as the "will to persevere," a phrase I think will be variously interpreted, but which seems apt enough for the current paper. However, one concern that popped into my head while reading through the article (dons reviewer glasses, purses lips disapprovingly) was: Is it really a will to persevere, or just a general increase in autonomic nervous system arousal (i.e., the sympathetic branch)? The "will to persevere" reported here may be the patient's interpretation of his increased heart rate, which, given the circumstances of the experiment and the patient's undergoing surgery to treat his epilepsy, may reflect his desire for a successful outcome of the surgery. Placing the patient in a different environment or with different circumstances - say, locking him in a room with one of the facehuggers from the movie Alien - may lead to a reinterpretation of the same increased arousal as fear, instead of a general willingness to overcome the challenge that lays in front of him.
In any case, these results, coupled with the lack of emotional response to electrical charges delivered to control stimulation sites and sham stimulations, lends support to the theory that the midcingulate region plays some kind of role in motivation, and that stimluation to this region may have practical applications for disorders involving pathologically low amounts of motivation, such as major depression and senioritis; disorders which, I might add, I am fully qualified to treat with open-brain surgery and a homemade electrical stimulation kit consisting of copper wire and a couple of lemons. Just give me a call.
Link to paper (including video of interview with subject 1; scroll to bottom of page): http://www.sciencedirect.com/science/article/pii/S0896627313010301