For any young cognitive neuroscientist out for the main chance, ROI analyses are an indispensable part of the trade, along with having a cool, marketable name, such as Moon Unit or Dweezil Zappa. Therefore, you will find yourself doing a lot of such ROI analyses; and the quicker and more efficient you can do them, with a minimum of error, will allow you to succeed wildly and be able to embark upon an incredible, interesting career for the next four or five decades of your life before you die.
Most ROI analyses in SPM are carried out through the Marsbar toolbox, and for most researchers, that is all they will ever need. However, for those who feel more comfortable with the command line, there is a simple command within the SPM library - spm_get_data - that will make all of your ROI fantasies come true. All the command needs is an array of paths leading to the images you wish to extract data from, along with a matrix of coordinates representing the ROI.
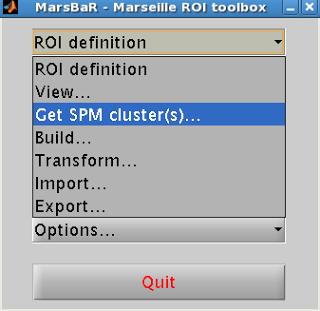
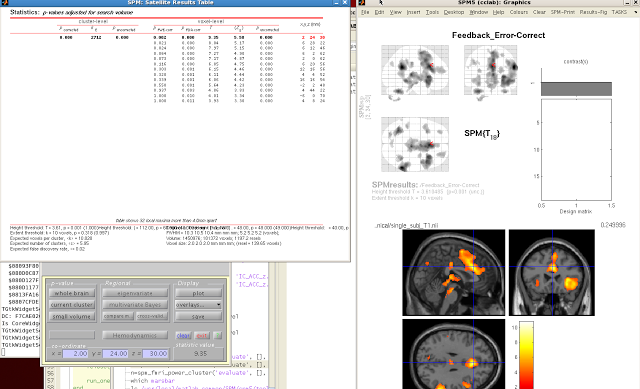
First, the ROI coordinates can be gathered by loading up an arbitrary contrast and selecting an ROI created through, say, Marsbar or wfu_pickatlas. Next, set your corrected p-value threshold to 1; this will guarantee that every voxel in that ROI is "active," which will be recorded by a structure automatically generated each time the Results GUI is opened, a structure called xSPM. One of the fields, xSPM.XYZ, contains the coordinates for each voxel within that ROI. This can then be assigned to a variable, and the same procedure done for however many ROIs you have. The best part, however, is that you only need to do this once for each ROI; once you have assigned it to a variable, you can simply store it in a new .mat file with the "save" command (e.g., save('myROIs.mat', 'M1', 'ACC', 'V1')). These can then be restored to the Matlab workspace at any time by loading that .mat file.
Note: An alternative way to get these coordinates just from the command line would be the following:
Y = spm_read_vols(spm_vol(seedroi),1);
indx = find(Y>0);
[x,y,z] = ind2sub(size(Y),indx);
XYZ = [x y z]';
Where the variable "seedroi" can be looped over a cell containing the paths to each of your ROIs.
The next step is to create your array of images you wish to extract data from - which, conveniently, is stored within the SPM.mat file that is created any time you run a second-level analysis. For example, let's say that I carried out a couple of 2nd-level t-tests, one for left button presses, the other for right button presses. If I go into the folder for left button presses that has already estimated an run a second-level analysis, all of the contrast images that went into that analysis are now stored in SPM.xY.P, which is available in your workspace after simply navigating to the directory containing your SPM.mat file and typing "load SPM".
Lastly, spm_get_data is called to do the ROI analysis by extracting data from each voxel in the ROI for each subject for each contrast, and these are averaged across all of the voxels in that ROI using the "mean" function. Sticking with the current example, let's say have a left M1 region and a right M1 region, the coordinates of which have been extracted using the procedure detailed above, and which have been saved into variables called left_M1 and right_M1, respectively. I then navigate to the left button presses second-level directory, load SPM, and type the following command:
right_M1_leftButtonPress = mean(spm_get_data(SPM.xY.P, right_M1),2)
which returns an array of one value per subject for that contrast averaged across the ROI. You can then easily navigate to another second-level directory - say, right button presses - and, after loading the SPM.mat file, do the same thing:
right_M1_rightButtonPress = mean(spm_get_data(SPM.xY.P, right_M1),2)
T-tests can then be carried out between the sets of parameter or contrast estimates with the t_test function:
[h, p, ci, stats] = ttest(right_M1_leftButtonPress, right_M1_rightButtonPress)
which will return the p-value, confidence interval, and t-value that you would then report in your results.